Overview of GANE model BBS paper
GANE Model
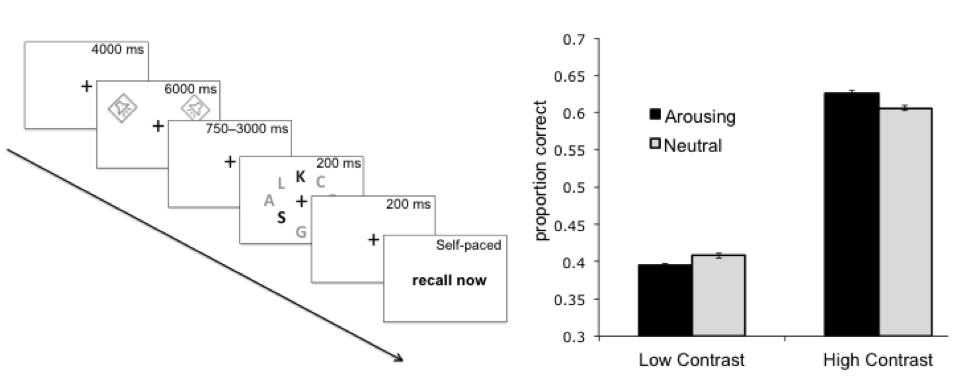
What happens to your cognitive processes when your arousal levels increase? Decades of research indicate that arousal impacts attention and memory, but a puzzling thing has been that it can have opposite effects in different experiments. Through a series of behavioral and MRI studies, we found that whether arousal will enhance or impair information processing depends on a simple factor: the priority of the information. When a stimulus stands out because of its salience or its goal relevance, its processing tends to be enhanced by a surge of arousal. In contrast, processing of lower priority stimuli tends to be impaired by arousal. For instance, consider the array of letters in the figure below (from Sutherland & Mather, 2012). If we play a neutral sound before flashing this array, participants tend to be able to report more of the dark grey letters than the light grey letters, because the dark grey letters are more salient and attract more attention. But if we play an arousing sound (such as a baby crying), this effect of salience increases: participants report even more of the salient letters and fewer of the non-salient letters.
That the effects of arousal depend on priority helps make sense of contradictory findings in the literature and provides a rubric to predict whether arousal will help or hurt your thinking processes. But it also raises another question: How can this work in the brain? How can the neurochemicals released during arousal “know” which neural networks to amp up and which ones to tamp down?
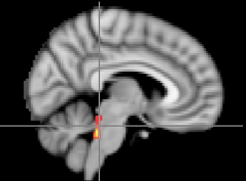
Questions about how arousal works in the brain bring us to the locus coeruleus, a small brainstem nucleus that plays a key role in fluctuations in arousal. Neurons in the locus coeruleus have long axons that project throughout most of the brain, allowing for broad release of noradrenaline from varicosities distributed along those axons whenever arousal levels increase — for instance when simply awake or active, or when thinking hard or hearing a sudden loud noise. Thus, activation of the locus coeruleus under arousal seems likely to have a broad diffuse effect. But a broad effect does not fit with our findings. For instance, arousal can simultaneously enhance representations of salient letters and impair representations of non-salient letters.
To address this puzzle, we proposed the Glutamate Amplifies Noradrenergic Effects (GANE) model. In this model, locus coeruleus neurons release low-to-moderate levels of noradrenaline throughout the brain when activated. These levels of noradrenaline are sufficient to activate alpha1 and alpha2 noradrenergic receptors that have relatively low thresholds for activation and often have inhibitory effects. Thus, under arousal, throughout most of the brain, neural activity quiets. However, where there are already high levels of activity, glutamate spills over from synaptic activity and activates receptors on nearby locus coeruleus axons. This stimulates more noradrenaline release, which stimulates more glutamate release via high threshold beta-adrenergic receptors, leading to a local hot spot of elevated activity. Thus, by amplifying excitatory activity while inhibiting most other activity, arousal helps focus attention on what really matters.
More details can be found in our paper:
Mather, M., Clewett, D., Sakaki, M., & Harley, C.W. (2016). Norepinephrine ignites local hot spots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behavioral and Brain Sciences. PDF
Commentaries
We were honored by the thoughtful commentaries on our target paper and excited to discover so many interesting links and connections.
Perspectives from leading experts on the neuroscience of norepinephrine
Abdallah, Chadi G.; Averill, Lynnette A.; Krystal, John H.; Southwick, Steven M.; and Arnsten, Amy F.T.
Glutamate and Norepinephrine Interaction: Relevance to Higher Cognitive Operations and Psychopathology
Bouret, Sebastien
For better or worse, or for a change?
Eldar, Eran; Cohen, Jonathan D.; and Niv, Yael
Amplified selectivity in cognitive processing implements the neural gain model of norepinephrine function
Gaucher, Quentin and Edeline, Jean-Marc
Are there “local hot spots?”: When concepts of cognitive psychology do not fit with physiological results.
Navarra, Rachel and Waterhouse, Barry
“What have we GANEd?” Application of a theoretical construct to explain experimental evidence for noradrenergic regulation of signal processing in sensory networks.
Roozendaal, Benno; Luyten, Laura; de Voogd, Lycia D.; and Hermans, Erno J.
Importance of amygdala noradrenergic activity and large-scale neural networks in regulating emotional arousal effects on perception and memory
Sara, Susan J.
Locus coeruleus reports changes in environmental contingencies
Strange, Bryan A. and Galarza-Vallejo, Ana
Bidirectional synaptic plasticity can explain bidirectional retrograde effects of emotion on memory
Todd, Rebecca M.; Ehlers, Mana R.; and Anderson, Adam K.
What BANE can offer GANE: Individual differences in function of hotspot mechanisms
Warren, Christopher Michael; Murphy, Peter Richard; and Nieuwenhuis, Sander
Cognitive control, dynamic salience and the imperative toward computational accounts of neuromodulatory function
Modulation by the heartbeat (reviews some amazing findings)
Critchley, Hugo D. and Garfinkel, Sarah
Bodily arousal differentially impacts stimulus processing and memory: norepinephrine in interoception
Modulation by cortisol
Hurlemann, Rene; Maier, Wolfgang; and Scheele, Dirk
Interactions of noradrenaline and cortisol and the induction of indelible memories
Other perspectives
These discussed GANE in the context of a variety of topics such as infancy, aging, dreams, memory consolidation, fluency, dentate gyrus of the hippocamus, intracellular mechanisms for amplifying signal, and event-related potentials
Becchetti, Andrea and Amadeo, Alida
Does the Glutamate Amplifies Noradrenergic Effects (GANE) model help explain why we fail to remember our dreams? Acetylcholine and norepinephrine balance in wakefulness and rapid-eye-movement (REM) sleep.
Carbon, Claus-Christian and Albrecht, Sabine
The Fluency Amplification Model supports the GANE principle of arousal enhancement
Ferreira-Santos, Fernando v
The role of arousal in predictive coding
Geva, Ronny
Contemplating the GANE model using an extreme case paradigm
Houghton, Conor
The Dentate Gyrus and the Hilar Revised
Hull, Thomas D.
GANEing on Emotion and Emotion Regulation
Huntsinger, Jeffrey R. and Storbeck, Justin
Once more with feeling: On the explanatory limits of the GANE model, and the missing role of subjective experience.
Kaspar, Kai
Arousal-biased preferences for sensory input: An agentcentered and multi-source perspective
Larkum, M. E. and Phillips, W. A.
Does arousal enhance apical amplification and disamplification?
Montagrin, Alison and Sander, David
Emotional memory: from affective relevance to arousal
Mouras, Harold
Effect of arousal on perception as studied through the lens of the motor correlates of sexual arousal
Nassar, Matthew R.; Bruckner, Rasmus; and Eppinger, Ben
What do we GANE with age?
Phaf, R. Hans
Competition Elicits Arousal and Affect
Ritchey, Maureen; Murty, Vishnu P.; and Dunsmoor, Joseph E.
Adaptive memory systems for remembering the salient and the seemingly mundane
Talmi, Deborah and Barnacle, Gemma E.
Emotionally arousing context modulates the ERP correlates of neutral picture processing: An ERP test of the Glutamate Amplifies Noradrenergic Effects (GANE) model
Response to Commentaries
Mather, M., Clewett, D., Sakaki, M., & Harley, C.W.
GANEing traction: The broad applicability of NE hotspots to diverse cognitive and arousal phenomena