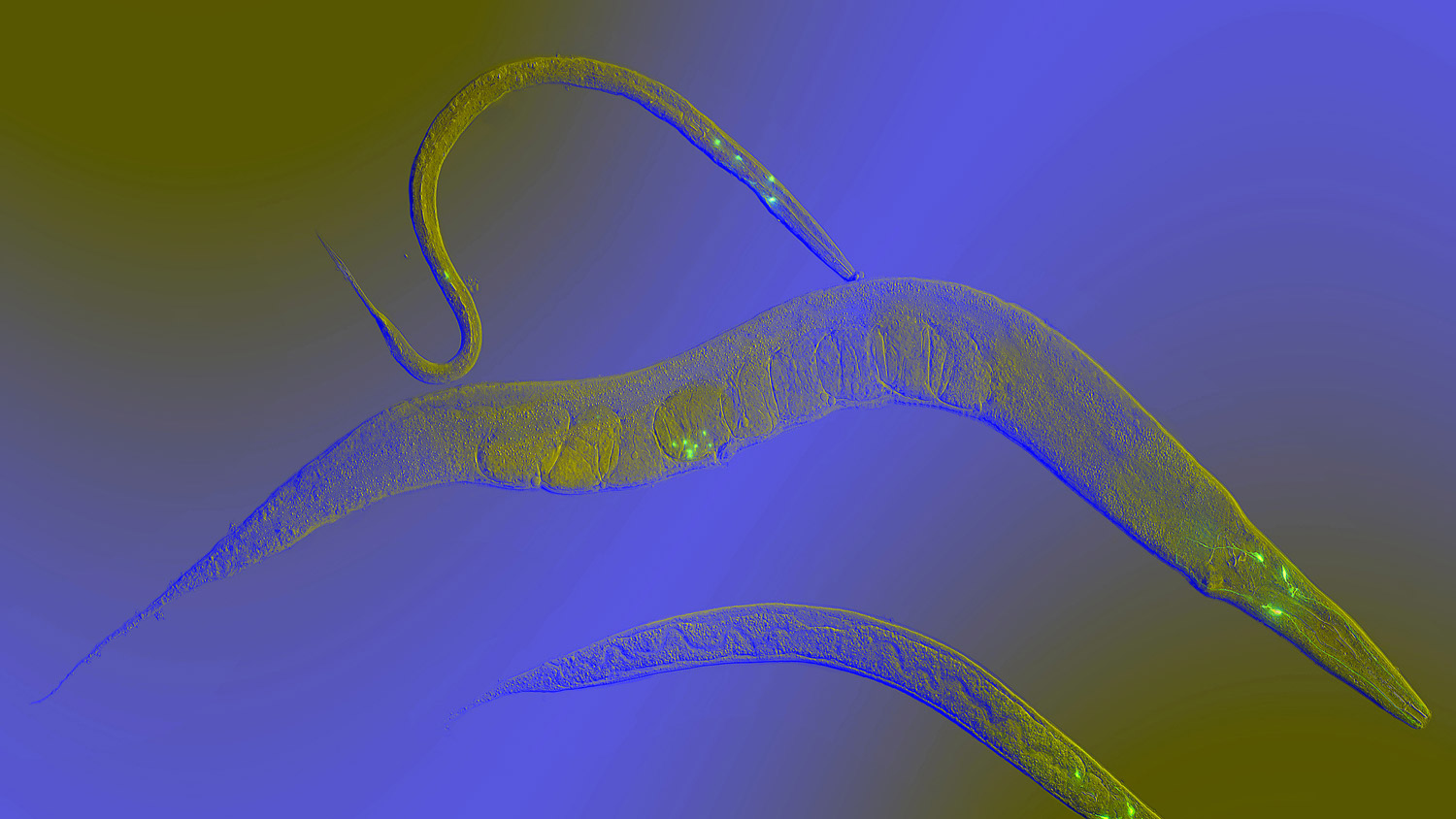
A new study by researchers at USC and UC Berkeley sheds light on how changes in lipid metabolism can impact the health of cells as they age and respond to stress.
The study focuses on the endoplasmic reticulum (ER), a structure within cells responsible for producing proteins as well as lipids, fatty compounds that play a variety of roles within the body. A properly functioning ER, sometimes called the “factory” of the cell, is essential for maintaining the quality of proteins and lipids within cells, said first author Gilberto Garcia, a postdoctoral researcher at the USC Leonard Davis School of Gerontology.
Garcia and colleagues identified a gene in the worm species C. elegans, let-767, that plays an important role in maintaining the quality of lipids and proteins produced by the ER. When the let-767 gene was suppressed, lipid stores in the worms’ cells decreased. The structure of the ER was altered, lessening its ability to respond to protein-related stress, and the affected worms were smaller and lived shorter lives.
“let-767 codes for an enzyme that gets rid of toxic lipids and then converts them into good lipids,” said Ryo Sanabria, assistant professor of gerontology at the USC Leonard Davis School and a coauthor of the study. “So if you get rid of it, then it really destroys ER health in two different ways: it accumulates the toxic product that it’s supposed to get rid of, and then also you don’t have the good stuff that it’s supposed to turn it into.”
Suppression of let-767 and the resulting depletion of lipids blocked induction of the Unfolded Protein Response of the ER (UPRER), a process typically triggered by the over-accumulation of unfolded or misfolded proteins. Normally, the UPR acts to refold or clear out the oversupply of improperly folded proteins.
The blockage of this response could play an important role in neurodegenerative conditions such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS), Sanabria said. These illnesses are examples of proteopathic diseases, which are characterized by the accumulation of abnormal proteins.
“This finding shows that changes in lipid pathways could significantly impact a cell’s ability to respond to protein stress and brings to light how a lipid imbalance could hasten the progression of proteopathic diseases,” they said.
The study, “Lipid homeostasis is essential for a maximal ER stress response,” appeared in eLife on July 25, 2023. Along with Garcia and Sanabria of USC, the study’s authors included corresponding author Andrew Dillin and coauthors Hanlin Zhang, Sophia Moreno, C. Kimberly Tsui, and Brant Michael Webster, all of UC Berkeley. This work was supported by the Biology of Aging training grant from the National Institute on Aging at USC, which supports Gilbert as a postdoctoral trainee. Additional sources of funding include additional grants from the National Institute on Aging, Larry L. Hillblom Foundation, and Howard Hughes Medical Institute.